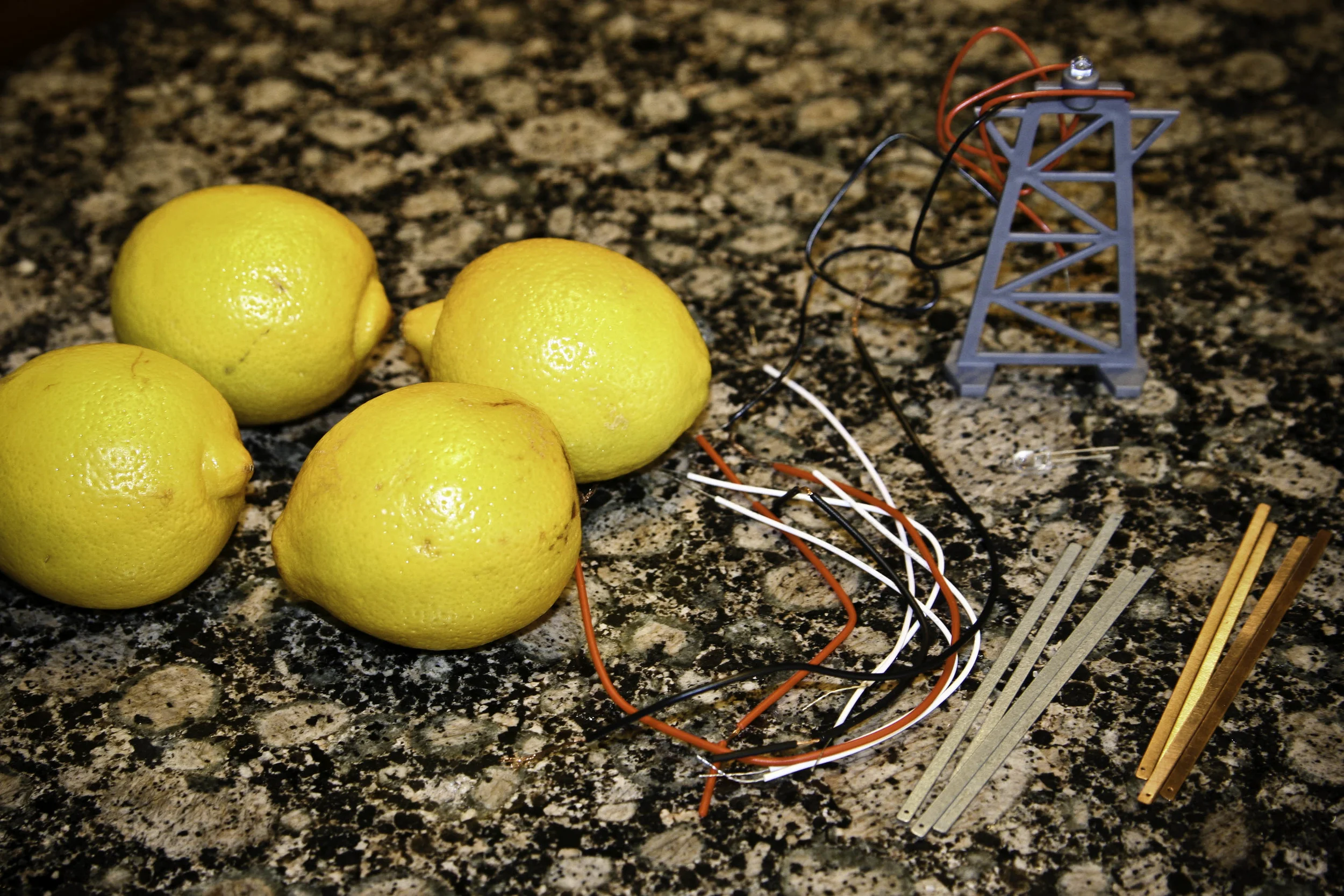
Lemon Battery Science Experiment
A Lemon Battery Science Experiment conducted by a 5th grader: A Lemon Battery Science Experiment creates an electrical current flow through the juice of the lemons and lights an LED bulb.
A Lemon Battery Science Experiment conducted by my 5th grader:
A Lemon Battery An electrical current flows through the juice of the lemons and lights the LED bulb.
What Do You Need? SUPPLIES
- 12-2-inch strip of copper
- 12-2-inch strip of zinc
- 12 Lemons
- 14-Alligator clip leads
- An electrical meter for measuring direct current volts in the 0-10-15-volt range.
- LED light bulb
- Help from an adult
What To Do? INSTRUCTIONS
1) Gently roll each lemon with your hands. But don't rupture the lemon's skin. This will increase electrical conductivity in each lemon.
2) Push one 2-inch strip of copper into the end of a lemon. Stop in the center of the lemon.
3) Push one 2-inch strip of zinc on the opposite end of the lemon. Stop in the center of the lemon. The lemon is now an electrolytic cell.
4) Do this for each lemon. The copper and zinc are now electrodes.
5) Attach a wire jumper lead to the copper of the first lemon to the zinc of the second lemon.
6) Next attach a wire jumper lead to the copper of the second lemon and then the zinc of the third lemon.
7) Continue until each lemon has been connected. When finished, the zinc in the first lemon and the copper of the last lemon are still unconnected.
WHAT HAPPENS?
Each lemon is now an electrolytic cell. When clipped together, the cells become a battery.
A sufficient amount of power has been created to light the LED.
Now that your lemon battery is ready, touch one jumper lead from one side of the meter to the zinc strip of the first lemon and the other jumper lead to the copper strip of the last lemon cell. As you walk through the battery from lemon cell to lemon cell, the voltage will increase.
The copper strip and zinc strips, which are the electrodes, are important in two ways. First, they conduct electricity. Second, the zinc coating of the nail is really fuel for the electrolytic cell. As it reacts in the cell, it changes from being metallic to being part of a molecule.
There's a chemical reaction between zinc and the lemon juice. There's also a chemical reaction between the copper and the lemon juice. These two chemical reactions push electrons through the wires. This flow of electrons is called an electrical circuit. This makes electricity, and with the help of the LED, it changed it into light.
Hypothesis
An electrical circuit can be made using the juice of lemons, copper and zinc strips and alligator clip leads.
Conclusion
The lemons did not work very well. The reason is that the lemons produce only a very small current. This is not enough electric current to light the LED well. Even with 12 lemons, the amount of current flowing through the wire is not enough. Though the voltage is high enough (8.5 volts with 12 lemons), the current is too weak to light the LED well. The 1st test did not light the LED at all. The 2nd test lit the LED for a short time. The 3rd test gave the best results, with the voltage increasing much more than I had thought. If a lemon or all the lemons are continually hooked to the meter, the voltage drops with time.